Status: Actively Enrolling Starting September/October 2020
Are you interested in a device that could potentially reduce the need for a diverting stoma, after your colon resection?
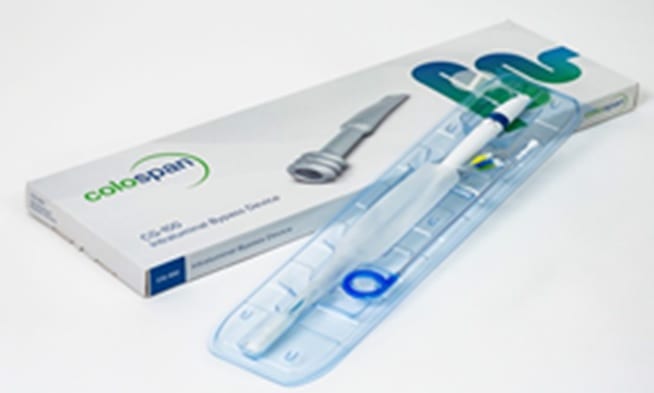
We are conducting a clinical research study to evaluate the safety and effectiveness of the investigational CG-100 Intraluminal Bypass Device in reducing the need for a diverting stoma. Patients that are undergoing a colorectal surgery with a mesorectal excision and are planned to have a protecting diverting stoma as part of their operation.
This study would potentially treat your condition using the CG-100 Intraluminal Bypass Device. The device is a protective intraluminal tube deployed at the anastomotic site to protect it from fecal contact in order to give the site time to heal. The CG-100 Intraluminal Bypass Device is a treatment option that could lower the likelihood that a patient will require a diverting stoma procedure. However, the CG-100 Intraluminal Bypass Device has not been approved by the FDA for use in treating colorectal anastomosis. Although the CG-100 Intraluminal Bypass Device is experimental for this treatment in the United States (US), it is expected that the CG-100 Intraluminal Bypass Device will allow you to avoid having a diverting stoma procedure, although this is not guaranteed. Your participation in this study will help us to test this hypothesis.
Candidates for Clinical Trial
You may qualify for this study if you:
-
- Diagnosed with rectal cancer, and need to undergo surgery
- Between ages 22-65 years old
Study Length & Information: 10.5 months, with a potential to follow up to 5 years postoperatively. The study sponsor will pay the costs for any study-specific tests that you will need as part of your participation in this study. If you agree to participate in this study, Colospan will also pay for the cost of the investigational treatment you receive during your operation (CG-100 Intraluminal Bypass Device)
Principal Investigator:
Research Nurse Contact:
Melanie Salerno
- Phone: (401) 450-2507
- Email: melanie.salerno@brownphysicians.org